Fujifilm announces the start of a phase III clinical trial of influenza antiviral drug “Avigan Tablet” on COVID-19 and commits to increasing production.
FUJIFILM Toyama Chemical Co., Ltd. (President: Junji Okada) has announced today the initiation of a phase III clinical trial to evaluate the safety and efficacy of influenza antiviral drug “Avigan Tablet” (generic name: favipiravir) in Japan for patients of COVID-19, a respiratory infection caused by the novel Coronavirus (SARS-CoV-2).
Following the rapid and global outbreak of COVID-19, on March 11 2020 the World Health Organization (WHO) announced the Coronavirus had become a global pandemic. As the number of COVID-19 patients continues to increase around the world, the development of effective treatments is urgently required.
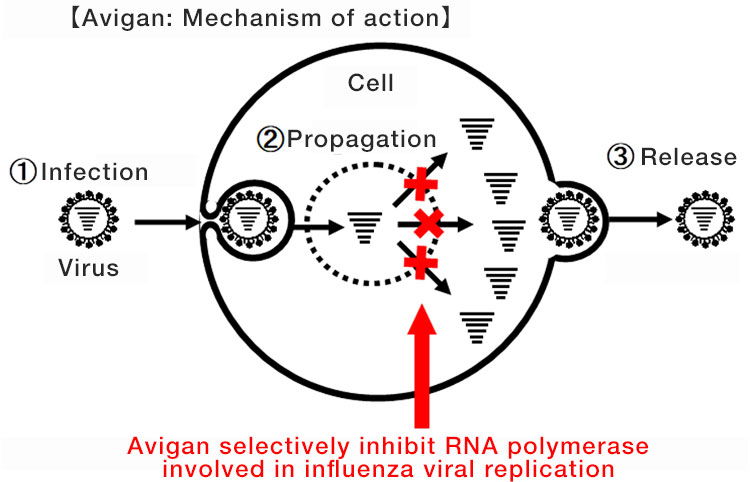
Avigan, approved for manufacture and sale as an influenza antiviral drug in Japan, has a mechanism of action for selectively inhibiting RNA polymerase involved in influenza viral replication. Due to this mechanism, it is expected that Avigan may potentially have an antiviral effect on the new coronavirus as it is classified into the same type of single-stranded RNA virus as influenza, and its clinical application to treat COVID-19 is now under study.
In early March, Fujifilm already began increased production of Avigan and now plan to accelerate the production of Avigan through cooperation with domestic and overseas partners for responding to the needs of the Japanese government and other countries.
Fujifilm intends to sincerely cooperate with the supply of Avigan to such countries in consultation and coordination with the Japanese government to combat COVID-19 and contribute to tackling the spread of this global pandemic at the earliest possible stage.